Envisioning Scientific Advances

From the earliest “flea glasses” of the first century to Leeuwenhoek’s microscope that opened a world beyond the naked eye in the 17th century, imaging has been a cornerstone of scientific discovery.
The microscope didn’t change much for nearly 200 years until improved technology enabled imaging to become a larger focus in medical research. Recent technological advances now allow scientists to witness cellular and developmental processes in real time—from single molecules and cells to complete tissues and embryos.
“Advanced imaging drives discovery in fundamental and translational research,” said Associate Professor Colin Reardon, director of the Advanced Imaging Facility. “A lot of questions in basic science involve spatial relationships of cells, how they change over their life span and how they respond to external challenges such as pollutants, toxic exposures and infection.
The microscope didn’t change much for nearly 200 years.
Observing those cellular interactions and expressions requires high-end microscopy. Thankfully, veterinary school scientists, as well as medical and biological sciences researchers at the university have access to three cuttingedge imaging tools at the Advanced Imaging Facility. Opened as a core campus resource in 2015, the facility is housed in a non-descript room of approximately 600 square feet on the main veterinary school campus. Users include participants in the Students Training in Advanced Research program, DVMs, graduate students, postdocs, and faculty members.
Advanced imaging drives discovery in fundamental and translational research.”
—Associate Professor and Advanced Imaging Facility Director Colin Reardon
“We’re able to support research endeavors from early to late-stage careers and also serve as an integral part of training for the next generation of researchers,” said facility manager Ingrid Brust-Mascher.

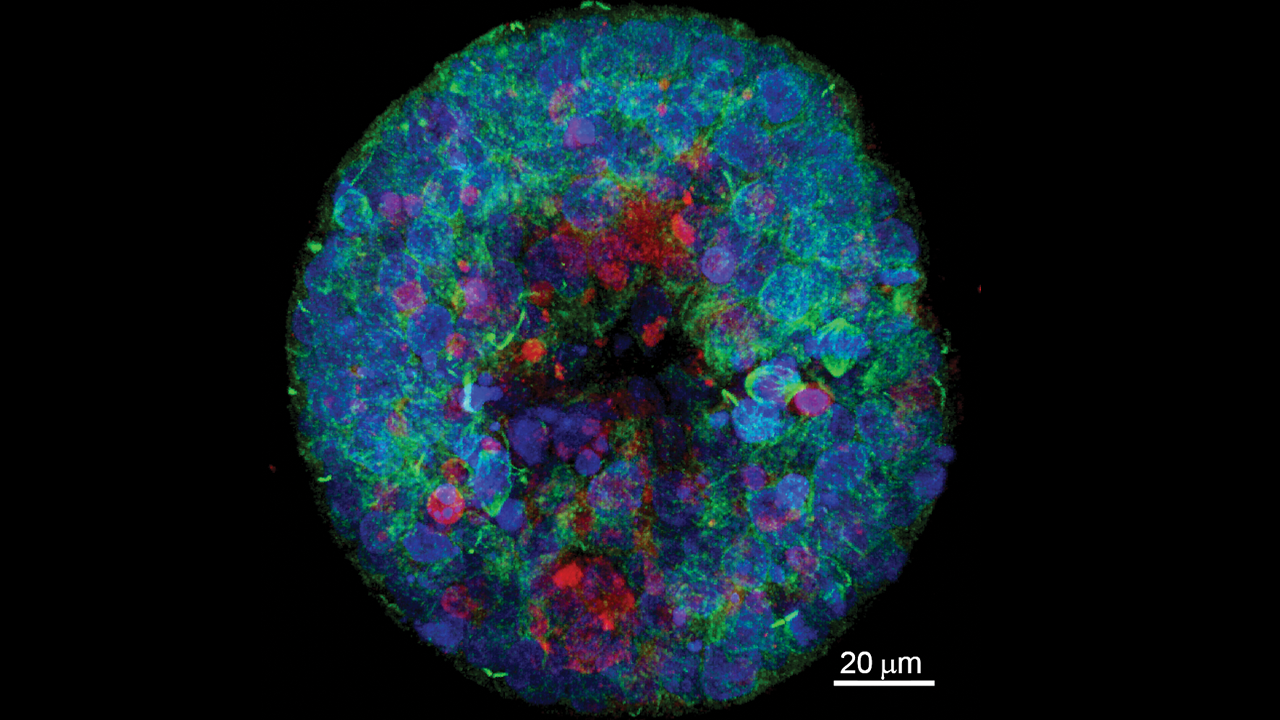
The images captured by the three specialized microscopes are anything but non-descript and more like elaborate abstract art to a non-scientist eye.
Two state-of-the-art Leica confocal microscopes (which allow confocal, super-resolution and multiphoton imaging) and a Zeiss lightsheet microscope can provide visuals from an eight-day equine blastocyst to a timelapse of microglia in a live zebrafish brain.

The Leica TCS SP8 MP Multiphoton Confocal Microscope with CLARITY allows researchers to peer deeply into intact biological specimens and reconstruct detailed 3-dimensional visual images of tissues and the cells within them. Faculty use this equipment to image brain, spleen or even the entire nervous system of a developing zebrafish embryo to answer questions about health and disease.
The Leica SP8 STED 3X Super-Resolution STED (STimulated Emission Depletion) Confocal Microscope permits researchers to view subcellular structures as small as 50 billionths of a meter (50 nanometers), allowing detailed visualization of cells, the structure of viruses and the interactions among individual proteins within cells.

The Zeiss Lightsheet 7 Microscopy unit is the latest addition and was purchased by a National Institutes for Health grant. It’s ideal for extremely fast and gentle imaging of whole living model organisms, tissues and cells as they develop over extended periods of time.
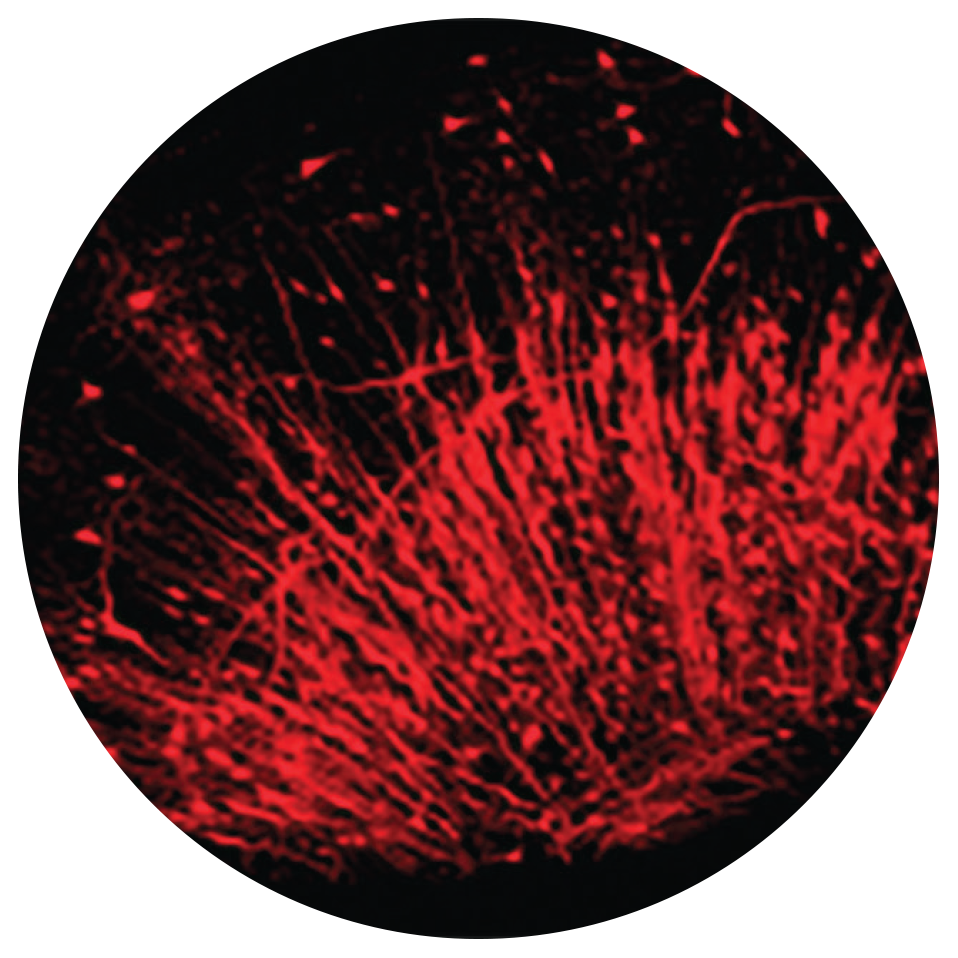
Microglia are a type of nerve cell located throughout the brain and spinal cord. They are also studied extensively for their harmful roles in neurodegenerative disorders—such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis.
Microglia are a type of nerve cell located throughout the brain and spinal cord. They account for about 10-15% of cells found within the brain and act as the first line of active immune defense in the central nervous system. They respond to pathogens and injury by changing morphology and migrating to the site of infection/injury, where they destroy pathogens and remove damaged cells. They are also studied extensively for their harmful roles in neurodegenerative disorders—such as Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis—as well as cardiac diseases, glaucoma, and viral and bacterial infections.
“Microglia are a definite theme here!” said Brust-Mascher. “We have the ability to look at a live, whole organism [such as the small zebra fish] and track cell behavior in real time. That allows researchers to conduct a longitudinal study of the same organism over time or span of treatments.”
Brust-Mascher’s Ph.D. in applied physics and her extensive experience in cell biology give her the ideal knowledge and background for her role. She has managed the facility since it opened nearly a decade ago and offers hands-on assistance to approximately 100 researchers from across the university who come to use the equipment each year. She provides consultation, training and support throughout an imaging experiment with preparation, acquisition and analysis.
We’re in a very unique position to have all three pieces of equipment in one location, specifically housed in a veterinary school.”
—Colin Reardon
While the initial expense of purchasing the equipment was supported by the dean’s office, additional funds from the campus’s Office of Research has paid for upgrades. In total, the facility houses close to $2.5 M worth of equipment and software. Users pay a recharge fee for access to the microscopes, which covers about 60-70% of annual operations. The remainder comes from other sources to pay for service contracts on equipment, software updates, etc.
“We’re in a very unique position to have all three pieces of equipment in one location, specifically housed in a veterinary school,” Reardon said. “Technologically advanced instrumentation is expensive and highly sensitive, requiring specialized training in order to promote their efficient and sustainable operation. We maximize the impact of such investments by providing shared access, and promoting new collaborations across disciplinary boundaries.”
For more information about the Advanced Imaging Facility, visit: advancedimaging.vetmed.ucdavis.edu or email ibrustmascher@ucdavis.edu
