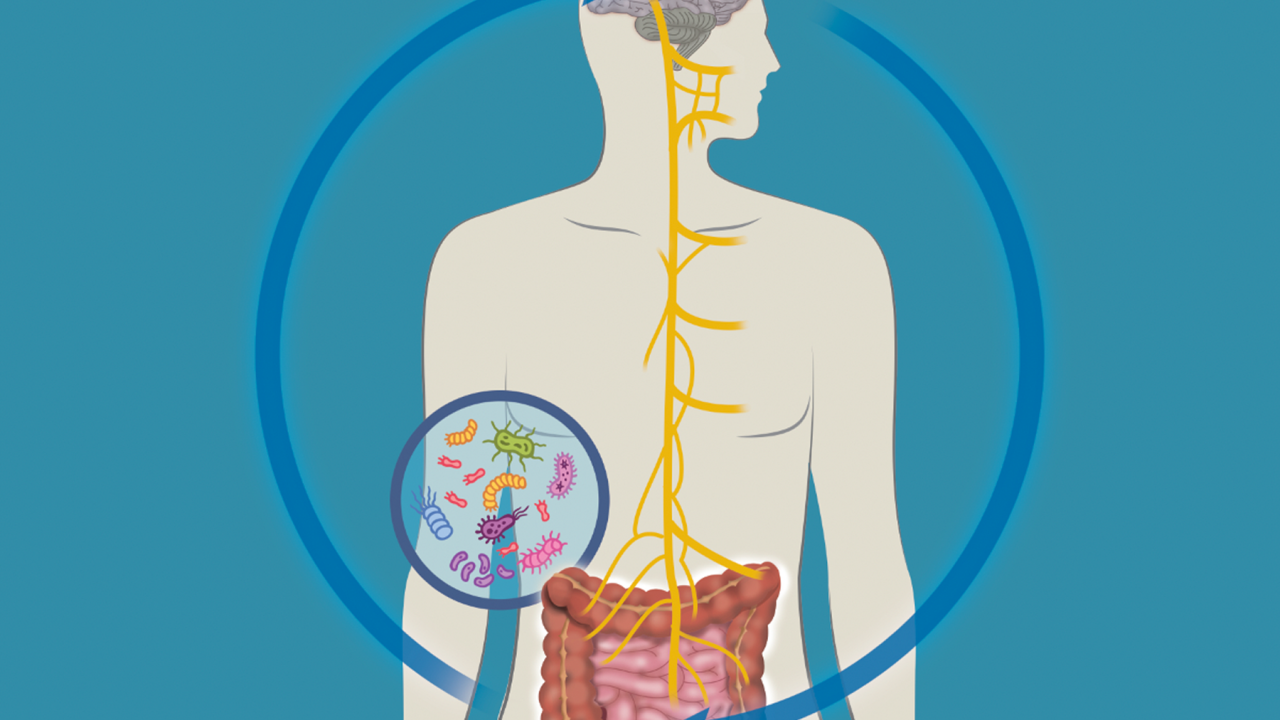
What’s Your Gut Got to Do With it?

hen you feel mental distress (anxious, stressed or nervous), it’s common to express that your stomach is in knots. It wasn’t until fairly recently that researchers began to understand this connection as a two-way street and to study how the gut also influences the brain.
This bi-directional communication pathway between the gastrointestinal (GI) tract and the central nervous system is now termed the microbiota-gut-brain (MGB) axis.
But how exactly do microorganisms in the gut—such as bacteria, fungi and viruses—impact communication between this organ and the brain? That is the big picture research question that Professor Melanie Gareau and Associate Professor Colin Reardon tackle on a daily basis at the school.
Combining Research Intersections
As a physiologist, Gareau seeks to understand how changes in the gut microbiome impact cognitive function, anxiety, and depression-like behaviors in mouse models. She has a particular interest in how microbes are dysregulated in conditions such as inflammatory bowel disease, bacterial infection and autism.
As a neuroimmunologist, Reardon has expertise in how the nervous system can alter the immune response. His research program relies on a number of light microscopy imaging modalities located in the Health Sciences District Advanced Imaging Facility for which he serves as director. The data sets produced by these techniques provide new opportunities to develop unique analytical tools and techniques.
Excitement Builds for Concept
Gareau, who was honored as a 2023 UC Davis Chancellor’s Fellow, was drawn to study the MGB axis two decades ago. Using a mouse model, she began looking at the impact of early life stressors on the development of mood disorders and altered intestinal function. That initial study, published in 2006 in Gut, clearly demonstrated the gut microbiome’s direct impact on behavior and became one of the seminal papers in the field. A few years later, a Swedish study bolstered that concept with more researchers studying the microbiome outside of their role in the gut.
“This concept went from being a very niche, almost esoteric idea, to the realization that this connection could impact many different aspects of physiology that have important implications on everyday health for many different patient groups,” Reardon said.
Gareau focused on how early life stressors impacted neurodevelopment in mice with the idea that kids facing trauma were more at risk of developing mood disorders, stress-related disorders, and PTSD in adulthood.
“The idea started forming that stress on the gut-brain axis early in life could basically alter the trajectory of neurodevelopment,” she said. “That's really what got me interested in the idea that kids were susceptible to this and that it could result in long-lasting defects.”
The flip-side question is whether that negative process can be mediated by modulating the microbiome by using probiotics to improve not only gut health but mental health.
She is currently exploring that question by using different models that disrupt the gut microbiome and seeing whether beneficial bacterial or beneficial bacterial metabolites can restore gut-brain communication.
From Stress to Infection
Gareau and Reardon met while in the same department for their Ph.D. studies at McMaster University in Canada and married in 2008. Reardon was interested in how the nervous system controlled the immune system in general and completed his doctoral training in a hospital environment with a strong gastroenterology department. He jokes that Gareau dragged him into neuroimmunology.
“I’d always wanted to go back to looking at the immune system in the gut,” he said. “There are lots of nerves there so it's a good opportunity to think about how those two systems talk.”
In the early days, many of their dinner table conversations revolved around the microbiota and bacteria and interfacing with the nervous system. It was helpful to bounce ideas off each other and brainstorm.
“That was before our son got older,” Reardon joked. “At 13, he gets pretty bored with it.”
With adjoining offices now less than 20 feet away, it’s easy to poke heads around the corner to share ideas and compare notes. As their research interests overlapped, Gareau and Reardon moved from looking at the impact of stress on gut health to the impact of infection, specifically whether infections alter gut-brain communication long term.
Reardon provides the example of a large percentage of children in the developing world or low- or middle income countries getting diarrhea from food and water-borne pathogens. Even after they receive oral rehydration which prevents more severe dehydration and death, there’s an add-on effect of failure to thrive at certain developmental milestones.
“I think that's a fundamental question and equally important even here in America,” Reardon said. “Food-borne outbreaks unfortunately impact children more severely and they can develop really bad consequences. Are those kids now set up to not meet their fullest potential in life? Is there something we could do about that?”
Multiple Communication Pathways
Establishing a link between gut health and brain function is one thing. Determining how it happens is another challenge.
“We’re trying to really understand how the gut communicates with the brain,” Gareau said. “We have lots of cool studies that suggest that they work together, but how do we pinpoint specifically what part of the gut talked to the brain?”
Technology allows researchers to study how neurons communicate within the brain over short distances in real time using functional MRI. But it’s a lot harder to measure activity between neurons from very distant organs such as the intestine.
“We don't have the necessary tools yet to adequately approach these scientific inquiries,” Reardon explained. “The gut nervous system is really complicated and poorly studied because it's much harder to characterize. And it moves! Which makes it more difficult to watch in real time on a microscope.”
It’s only in the past few years that technology has helped illuminate the different types of nerves that are present within the gut. The field is starting to break open and Reardon anticipates many more advances in terms of circuit-level analysis and understanding of what these cells are doing. One of the new techniques used to visualize nerve cell activation in the gut is known as optogenetics. It uses light to control neuronal activity in living tissue.
“Essentially you shine the light of a specific wavelength on a cell and the channel will open if it expresses that channel,” Reardon said. “It’s like you're clicking a flashlight on and off. The cool part is if you pulse the light, you can trick the cell into thinking it's receiving electrical input from another nerve, and then activate whatever downstream effects it's going to have. You can turn nerves off in the same kind of way using different wavelengths of light.”
These advanced technologies enabled by federal research funding will be key to further understanding and developing the next generation of therapies, Reardon added.
Wider Applications
Veterinary medical research into the MGB connection is lagging compared to human medicine, Gareau said. But there are a lot of opportunities moving forward as scientists discover more connections between gut health and behavior. For example, could veterinarians deal with problematic pet behaviors with probiotics or food if they better understood what components of those are important for regulating neurotransmitters?
“Or maybe clinicians include looking into whether an animal had an infection early in life that may predispose them to unwanted behaviors,” Reardon added. “We don’t even understand that in mice all the time so in a companion animal population, that’s a big question.”
Other outstanding questions for our pets include the roles of pre or probiotics.
“We use antibiotics a lot in both veterinary and human medicine and we haven’t spent a lot of time to determine whether we should be administering that with probiotics as a preventive to wiping out the gut microbiome, which includes healthy bacteria,” Gareau said. “If an animal has received high-dose antibiotics due to a procedure, does that predispose them to a greater risk for behavior issues?”
Just because a person or animal doesn’t die from an infection doesn’t mean that there can’t be other consequences, Reardon pointed out. “We need to understand these things from a systems level to really know what’s at risk in the future and potentially work toward solutions.”
“We have scientific consensus that gut health is important to brain health. Now we need to fully understand how the microbiome works to regulate this communication,” Gareau said. “Our microbiomes have changed so much in the past 50 years with additives and emulsifiers in foods, dyes, all the ingredients that we can’t read on the labels, and chemicals in our daily environments—they are definitely playing a role in our gut environment. What is the actual real risk from changes to our environment and how will that change our microbiome and our health in the long run? That’s a big unknown, but maybe we need to take better care of our microbiome so that we can improve our overall health.”
